
Exactech Knee Recall
The U.S. Food and Drug Administration (FDA) announced a recall of Exactech knee replacements with polyethylene components and inserts, due to risk of device failure that could lead to health risks and require corrective revision surgery. Exactech continues to expand its recall of its OPTETRAK Comprehensive Knee Systems that were packaged in certain faulty vacuum bags. They warned surgeons that even more devices may be impacted by the defective bags than the company originally thought.
In our post about the Exactech Recall Lawsuit we discussed the entire group of faulty implants. In today's post, we are focusing on the Exactech Knee Recall.
Issues With The Knee Implant
Exactech Knee Recall
Those with a knee implant subject to the Exactech knee recall may find that their replacement knee does not withstand normal wear-and-tear as expected.
Symptoms of a faulty knee implant include:
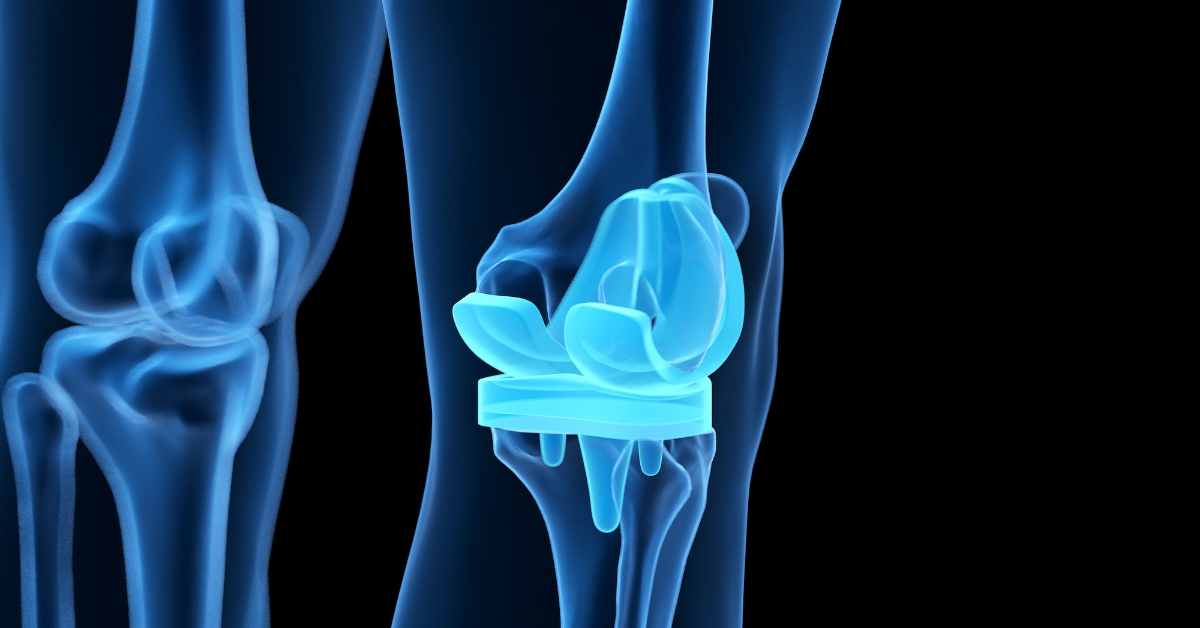
What is Knee Replacement vs Knee Revision Surgery?
The primary goal of both Knee Replacement and Knee Revision surgery is to relieve a patient’s pain, improve the function of their knees and their quality of life.
A standard total knee replacement (TKR) is made up of four parts:
- The femoral component – a metal piece that attaches to the thigh bone
- The tibial tray – a metal piece that fits into the shin bone
- The patellar component – a piece of plastic that fits onto the kneecap
- The tibial polyethylene insert – a plastic insert that fits between the femoral component and the tibial component, and acts as the new cushion for the replaced knee joint.
After the TKR surgery, issues may arise with the tibial polyethylene insert if the surgeon used one of the defective components from Exactech resulting in the need for revision surgery.
Knee revision surgery is necessary when an old knee replacement device fails. A device could fail for different reasons including an adverse immune response, infection, unexplained loosening of the implant, and scar tissue build up. Revision surgery is often more complicated and difficult to perform due to the unpredictability of removing an old joint replacement. Deteriorated implants can lead to infection in the bone, further complicating surgery.
What if I Have a Recalled Exactech Knee Implant?
Your doctor may refer you to the Exactech-Broadspire Helpline for further assistance if it is determined that you need revision surgery. However, this course of action will only initiate Exactech’s settlement claims process for out-of-pocket surgery costs. You will not be compensated for other losses like lost wages or pain & suffering. Therefore, it is advised that you first contact a specialized personal injury attorney before reaching out to Exactech directly.
If you aren’t sure if your knee replacement implant is subject to the Exactech recall, contact your doctor. They will be able to look up the serial number of your implant and confirm whether or not you have an affected Exactech implant.
The Attorneys at Falcon Law Firm are Here to Help

